Thousands of lives across the country will be changed forever as a new sickle cell drug, Voxeletor, has been approved for use in the NHS following a recommendation from the National Institute for Health and Care Excellence (NICE).
Voxeletor will be administered as a daily tablet. In England, an estimated 17,000 people are living with the disease, and around 4,000 will be eligible to receive the new treatment.
What is sickle cell disorder?
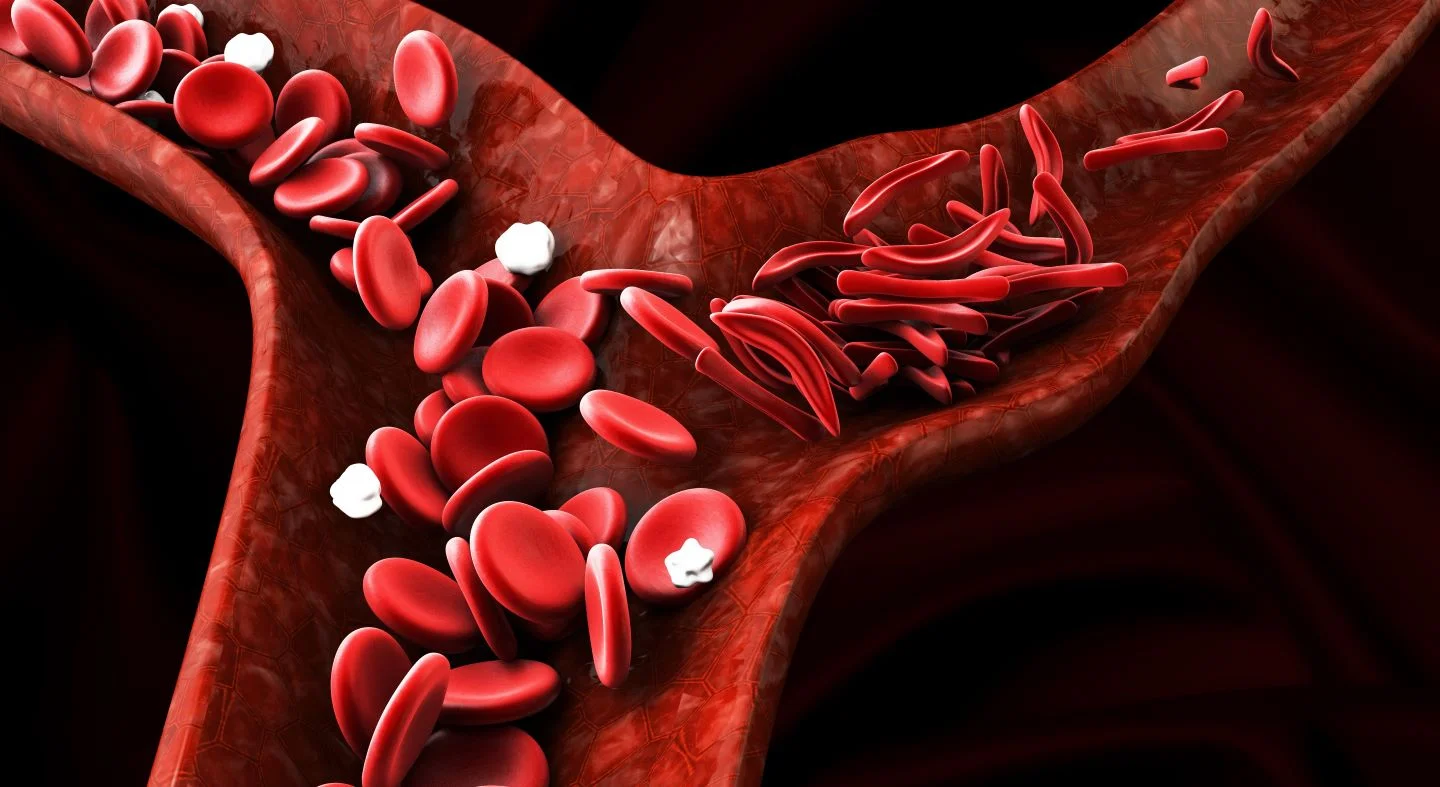
Sickle cell disorder causes red blood cells to assume a “sickle” shape, leading to premature breaking and death, significantly reducing their ability to carry oxygen. This condition results in chronic anemia and episodes of severe pain, called crises, which can be life-threatening and often require hospitalization. Furthermore, individuals with this inherited condition may experience various life-altering complications such as stroke, chronic fatigue, delayed growth, infections, and progressive tissue and organ damage. Voxeletor works by aiding hemoglobin, a protein in red blood cells, in retaining more oxygen and preventing red blood cells from becoming deformed.
Hazel Attuta shares her reaction to the drug with Sky News
Sky News reported on Hazel Attuta, a young woman who has been taking the medication for the last two years. She recalled how she used to work in finance but had to quit due to the chronic pain her illness caused. She said: “One of the most significant changes I’ve experienced is in my energy levels. Previously, the exhaustion from sickle cell made it challenging to function, impacting my ability to work and enjoy time with loved ones. I wasn’t living the life of a 20-something by any means. Since starting Voxeletor, my energy has soared, making a massive change to my daily routine. This has not only improved my physical well-being but also my mental health.”
Charities and Organisation welcome the approval of the drug with congrats and celebrations
Charities and organisations working closely with individuals living with sickle cell disease have welcomed the approval of the drug. Charles Kwaku-Odoi, CEO of the Caribbean and African Health Network (CAHN), expressed his delight, saying: “We are absolutely delighted that this treatment has been approved by NICE on behalf of people living with Sickle Cell and the Black community as a whole.
This treatment offers significant hope of a better quality of life for those suffering from this often-debilitating condition. At CAHN, we continue to advocate and amplify patient voices in addressing systemic inequities for a community that is underserved.” John James, CEO of the Sickle Cell Society, stated that NICE’s decision has brought “a great deal of hope,” and they are profoundly grateful for this development. “It is a deeply life-changing and celebrated moment for people living with the condition,” he added.
Thank you for reading, click the link below to read more of our Health Articles:



Leave a Reply
You must be logged in to post a comment.